Hydroxyethyl Imidazolines are produced by reacting AEEA (Aminoethylethanolamine) with fatty acids or oils (1:1) producing an amide and then cyclizing to produce a 5 member ring containing 2 nitrogens with an ethyl hydroxyl group attached. The R group attached to the ring will be determined by the fatty acid or oil used.
Hydroxyethyl Imidazolines are regarded as the most hydrophilic imidazolines compounds, the hydrophobicity will increase as carbon length decreases. Find use in the lubricant, paints & coatings, inks and road making industries, promoting adhesion rheology modification.
Salting the un-neutralized imidazoline with lower molecular weight acids (acetic, HCL or phosphoric) will make the imidazoline more water soluble. Salting with longer chain organic acids will make the imidazoline more oil soluble. Acid salts offer: wetting, emulsification, detergency, thickening, moisture displacement, corrosion inhibition, film forming and anti-static effects.
The un-neutralized imidazoline can be quaternized to produce a cationic surfactant, used in corrosion protection and emulsification, it allows for the formed corrosion inhibitor to have excellent film forming characteristics on metal, plastic or textiles.
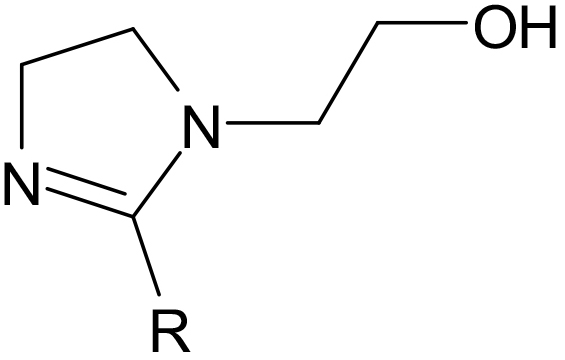
A hydroxyethyl Imidazoline (1:1) of Myristic acid (C14).
Appearance: Waxy solid
Amine Value: 200-280 mg KOH/g
Solubility: Oil soluble and water dispersible
Min % Imidazoline: 60
Acid Value: <5 mg KOH/g
Moisture: <0.1%
Melting pt.: 39°C
APPLICATION: Used to improve wetting, emulsification, detergency, corrosion inhibition, water displacement. Has antistatic and film forming properties.