A chemical compound derived from an acid (organic or inorganic) in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group. Esters are also usually derived from carboxylic acids. It may also be obtained by reaction of acid anhydride or acid halides with alcohols or by the reaction of salts of carboxylic acids with alkyl halides.
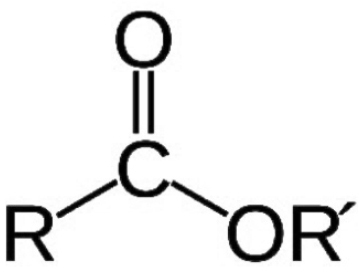
Monoisostearate (C18) esters of Polyethylene Glycol (PEG) of various molecular weights. They are soluble in water. Monoisostearate PEG esters improves emulsifier, wetting and lubrication properties. They are used in shampoos, hair conditioners and lotions. Remain liquid at room temperature.
HLB: 10.3
pH1% 5.0 – 7.0
Appearance: Clear pale yellow liquid
Acid Value: <3 mg KOH/g
Solids: >97%
Solubility: Water soluble
APPLICATIONS: Good wetting and dispersing properties. Emulsifier and adds lubricity. Improves cleaning.
Bring our expertise to your business. Let’s build valuable bonds together.