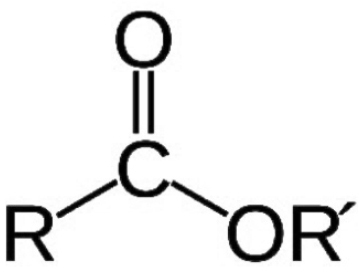
A chemical compound derived from an acid (organic or inorganic) in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group. Esters are also usually derived from carboxylic acids. It may also be obtained by reaction of acid anhydride or acid halides with alcohols or by the reaction of salts of carboxylic acids with alkyl halides.
Polyethylene Glycol (PEG) of various molecular weights with Oleic acid. The lower molecular weight versions are oil soluble, the higher molecular weight versions are water soluble, all are water dispersible. Monooleate PEG esters are good replacements for Nonylphenols (NP). They have low volatility and are very effective wetting agents. They are good emulsifiers and co-emulsifiers, able to aid in lubrication.
HLB: 15.9
pH1% 5.0 – 7.0
Solubility: Water soluble
Appearance: White solid
Acid Value: <5 mg KOH/g
Solids: >98%
Melt point: 35°C
APPLICATIONS: Effective wetting agent, viscosity modifier. Alternative to Nonylphenols (NP14). Used as surfactant in cleaners, lubricants, greases and metalworking fluids.
Bring our expertise to your business. Let’s build valuable bonds together.