Aminoethyl Imidazolines are produced by reacting DETA (Diethylenetriamine) with fatty acids or oils producing an amide and then cyclizing to produce a 5 member ring containing 2 nitrogen’s with an ethyl amine group attached. The R group attached to the ring will be determined by the fatty acid or oil used. The un-neutralized imidazoline will be lipophilic making them soluble in mineral oil or solvents and water dispersible.
Aminoethyl Imidazolines are good emulsifiers, will impart good lubrication (depends on R group). It is an excellent base for corrosion inhibitor formulations. Can be used as an intermediate for forming imidazoline surfactants and emulsifiers. Has good film formation. Determine solubilities in your formulation to best determine which imidazoline to use.
Salting the un-neutralized imidazoline with lower molecular weight acids will make the imidazoline more water soluble. Salting with longer chain fatty acids will make the imidazoline more oil soluble. The salts can be used for emulsification, corrosion inhibition, antistatic and detergency.
The un-neutralized imidazoline can be quaternized to produce a cationic surfactant, used in corrosion protection and emulsification, it allows for the formed corrosion inhibitor to be partitioned into the water cut and then form a tenacious film on the metal surface.
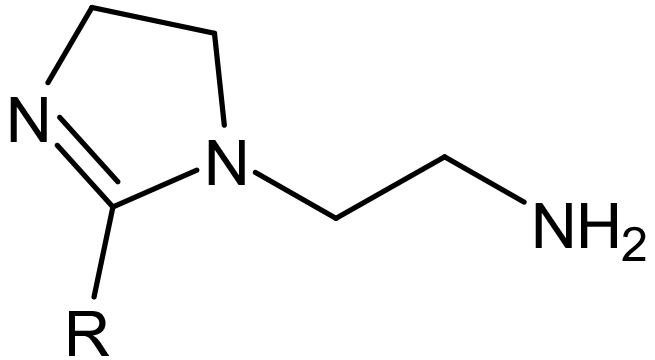
An aminoethyl Imidazoline (1:1) of Tall oil.
Appearance: Clear amber liquid
Amine Value: 180-224 mg KOH/g
Solubility: Oil soluble and water dispersible
Min % Imidazoline: 45
Acid Value: <10 mg KOH/g
Moisture: <0.1%
APPLICATION: Prevents corrosion in oil pipelines under high shear stress.
Bring our expertise to your business. Let’s build valuable bonds together.